Visualization of Electrochemical Behavior under Finite Conditions using JAVA and its Application for Assisted Learning
Hidenobu SHIROISHI, Tomoyo NOMURA, Kazunori ISHIKAWA, Sumio TOKITA and Masao KANEKO
 Return
Return
1 Introduction
Polymer-coated electrodes dispersing functional molecules have been studied during the last two decades [1 - 4]. These electrodes have wide application such as chemical sensors [5], electrocatalysis [6], and energy conversion devices [7]. It is important to study the charge propagation in polymer membranes for developing high-performance devices.
Needless-to-say a polymer layer coated on an electrode has a finite thickness. This causes a discrepancy in electrochemical results between the finite condition and the infinite condition from which equations in the solution system are derived. Another difference between these two systems is that the diffusion of molecules in a polymer layer is much slower than that in a solution. The contribution of the charge hopping mechanism to the whole charge propagation in a polymer-coated electrode would become greater than that in a solution system, so that equations in a solution cannot be applied to the polymer-coated electrode system depending on the situation. Under such finite conditions, it is difficult to solve diffusion equations, when the condition is altered a little. Thus an environment to analyze an electrochemical behavior easily and simply is needed.
In an educational respect, it is a problem to teach electrochemistry attractively because of a lot of abstruse equations. It is instructive to make an environment where every student can perform virtual electrochemical measurements using a network. Such a program has to satisfy the following requirements.
-
Calculations should be performed in each computer to prevent the concentration of calculations on the server.
-
The program should be distributed to each computer easily.
-
The program should be platform-independent for wide use.
JAVA is the best language to meet the above conditions.
In the present paper, we have developed a platform-independent program, called ES-1 (Electrochemical Simulator), written in JAVA language, which can calculate electrochemical behavior under finite conditions. This program is intended for a beginner using a modified electrode.
2 Method
2. 1 Theory of the Electrochemical Simulation
Figure 1 shows the scheme of a polymer-coated electrode in which functional molecules were dispersed. In an electrode-coated polymer layer, functional molecules (redox center) are randomly dispersed. The electrode is dipped in an electrolyte solution. Electrochemical measurement is performed by the conventional three electrode system. A conventional diffusion equation including first-order catalytic reaction was used for the simulation:

where C( x, t) (mol cm-3) is the concentration of the oxidized molecule at the time t (s), k (s-1) is the first-order rate constant for the catalysis by the oxidized molecule, D (cm2 s-1) is the diffusion coefficient of the charge. Assuming that a functional molecule(M) can catalyze a reaction, the first-order catalytic reaction is expressed by

where Mox and Mred are the oxidized and reduced forms of the redox center, respectively, S is a substrate, P is a product.
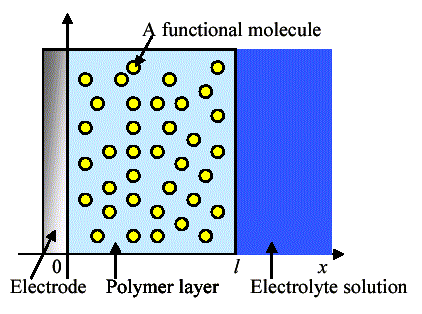
Figure 1. Scheme of a polymer-coated electrode with dispersed functional molecules
Assuming that the concentration of the redox species obeys a Nernstian equation [8], a boundary condition on the electrode (x = 0) is represented as
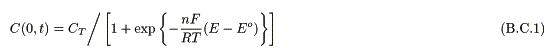
where CT (mol cm-3) is the total concentration of the molecule, E is the applied potential on the electrode, E° is the redox potential of the molecule, n is the number of electrons, F (C mol-1) is the Faraday constant. Another boundary condition under finite conditions is represented as

where l is the thickness of the polymer layer. A finite differential method (FDM) was used for the simulation.
2. 2 Implementation
We used a PC-9821 machine (NEC) in which Microsoft Windows 2000 was installed for developing ES-1 with the Microsoft Visual J++ version 6(SP3). However, we didn't use a Windows Foundation Class library (WFC) to keep a platform-independent feature. ES-1 was tested using IBM/AT compatible with Internet Explorer version 5.5.
3 Results and Discussion
3. 1 The Feature of ES-1
Figure 2 shows the combination of the ES-1 and electrochemical texts written in HTML. Students can learn electrochemistry by electrochemical text blended with the simulation program smoothly[9]. ES-1 has only one button in the control panel. Simple operation is very important for use in an electrochemistry class because most of the time in class should be spent for teaching electrochemistry itself, and not for teaching the usage of the program. The numerical results are put into a text area since JAVA applets are prohibited from accessing any local disks. The results in the text area can be copied by using a shortcut key ([Ctrl]+[C]).
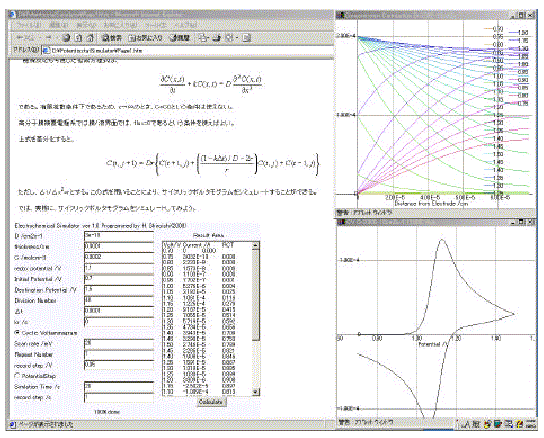
Figure 2. Combination of the ES-1 and electrochemical texts written in HTML. Upper window illustrates the concentration distribution of the oxidized molecule. Lower window shows the cyclic voltammogram of the molecule.
3. 2 Results of the Simulation
Figure 3 shows the time dependence of RCT estimated by absorption spectral change at potential step measurement using ITO/Nafion[Ru(bpy)32+] electrode. A curve calculated with ES-1, shown in Figure 3, coincides with the actual measurement suggesting that the simulation is reasonable. The diffusion coefficient used in the simulation was estimated by conventional equation in the solution system in the initial time region.
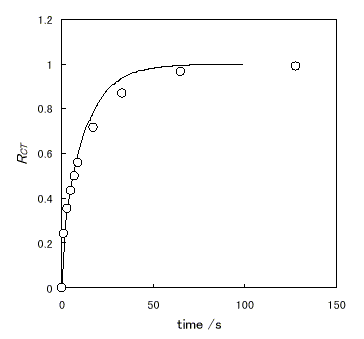
Figure 3. Time dependence of RCT estimated by absorption spectral change at potential step measurement from 0.6V (versus Ag|AgCl) to 1.4V using ITO/Nafion[Ru(bpy)32+] electrode in 0.1mol dm-3 KNO3 (pH 1). The solid line was calculated with ES-1. ( D = 3.2 × 10-10 cm2s-1, l = 1 × 10-4 cm, CT = 4.2 × 10-4 mol cm-3 )
Students can see the electrochemical behavior shown below using ES-1. Figure 4(a) shows a series of cyclic voltammograms at various thicknesses of a finite layer. The increase of the layer thickness raised the anodic current beyond the potential at the peak current, where the anodic current is derived from the diffusion of charges, but reduces the cathodic current on the reverse scan. This is because a thinner layer makes a steeper concentration gradient of the redox molecules.
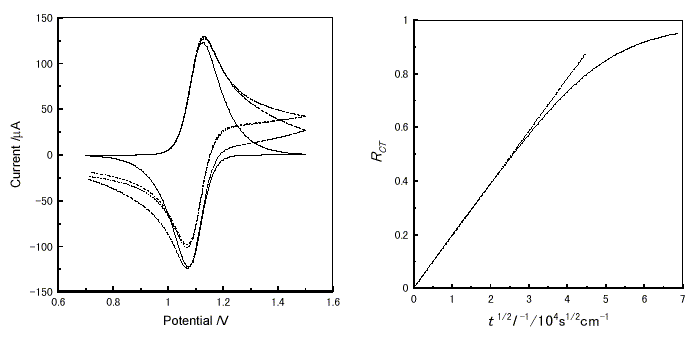
Figure 4. Virtual electrochemical measurements of a material (D = 3×10-10cm2s-1, k = 0 s-1, E° = 1.1 V vs. a standard electrode) at various thicknesses using ES-1. (a) Cyclic voltammogram from 0.7 V to 1.5 V at 20mV/s. -, l = 0.5 ×10-4 cm; - - -, 1.0 ×10-4 cm ; ..., 2.0 ×10-4 cm ; - . - 3.0 ×10-4 cm. (b) The plots of RCT vs. t1/2l-1 at applied potential from 0.7V to 1.5V. -, A simulated curve under finite condition; - - -, calculated by eq.3 (under infinite condition).
The equation of the time dependent RCT value under infinite conditions is represented by eq. 3 [10, 11], where RCT is the fraction of the redox molecule that accepted a charge.

Figure 4(b) shows the plots of RCT vs. t1/2l-1 simulated by ES-1. The simulated curve under the finite conditions deviated from the curve calculated by eq. 3 above RCT  0.5.
0.5.
Figure 5(a) shows a series of cyclic voltammograms at various k values. The anodic current beyond the redox potential increased with the k value. Time dependence of RCT under finite conditions at various k values in potential step measurement is shown in Figure 5(b). The increase of k value reduced the time to reach the plateau, and also lowered the plateau value.
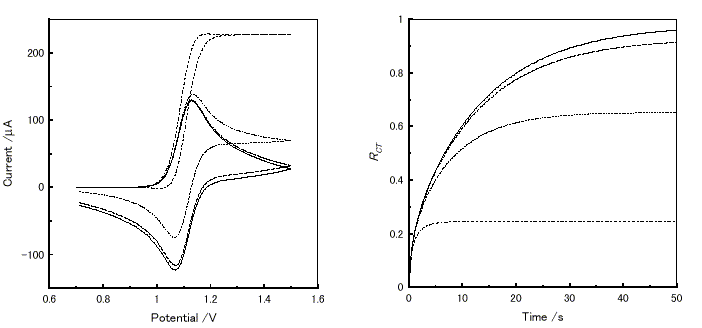
Figure 5. Virtual electrochemical measurements of a material (D = 3×10-10cm2s-1, E° = 1.1 V vs. a standard electrode) at various k values using ES-1. -, k = 0 s-1; - - -, 5 ×10-3 s-1 ; ..., 5 ×10-2 s-1 ; - . - 5 ×10-1 s-1. (a) Cyclic voltammogram from 0.7 V to 1.5 V at 20mV/s. (b) Time dependence of RCT at an applied potential from 0.7V to 1.5V.
4 Conclusion
An electrochemical simulator (ES-1) under finite conditions for redox centers confined in an electrode coated polymer layer was developed for an electrochemistry class. ES-1 was written in JAVA language which has characteristics of platform-independence, an easy cooperation with HTML and small load on a server computer. The RCT value under finite conditions deviated from that under infinite conditions above RCT  0.5.
0.5.
The authors acknowledge a Grant-in-Aid for JAERI's Nuclear Research Promotion Program (JANP) from Japan Atomic Energy Research Institute.
References
[ 1] K. Shigehara, N. Oyama, and F. C. Anson, J. Am. Chem. Soc., 103, 2552 (1981).
[ 2] C. R. Martin, I. Rubinstein, and A. J. Bard, J. Am. Chem. Soc., 104, 4817 (1982).
[ 3] J. S. Facci, R. H. Schmehl, and R. W. Murray, J. Am. Chem. Soc., 104, 4959 (1982).
[ 4] A. Aoki, R. Rajagopalan and A. Heller, J. Phys. Chem., 99, 5102 (1995).
[ 5] G. E. Benedetto, F. Palmisano and P. G. Zambonin, Biosensors & Bioelectronics, 11, 1001 (1996).
[ 6] M. Yagi, K. Kinoshita, and M. Kaneko, J. Phys. Chem., 100, 11098 (1996).
[ 7] M. Kaneko, Photoelectric Conversion by Polymeric and Organic Materials, Organic Conductive Molecules and Polymers, ed. by H. S. Nalwa, John Wiley & Sons, Ltd. (1997), vol. 4, p. 669.
[ 8] A. Fujishima, M. Aizawa, and T. Inoue, Denkikagakusouteihou, Gihoudou Syuppan Co., Ltd. (1984).
[ 9] This program embedded in HTML can be seen at
http://klab01.sci.ibaraki.ac.jp/~kanekolab/electrochem.html
[10] A. J. Bard and L. R. Faulkner, Electrochemical Methods - Fundamentals and Applications, John Wiley & Sons, New York (1980).
[11] P. Bonhote, E. Gogniat, T. Sophie, C. Barbe, N. Vlachopoulos, F. Lenzmann, P. Comte, and M. Gratzel, J. Phys. Chem. B, 102, 1498 (1998).
 Return
Return

 Return
Return


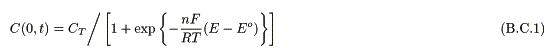





 0.5.
0.5.
 0.5.
0.5. Return
Return