Conformational Analysis of Hexagonal Arrangements
of Phosphatidylcholine Head Groups with Bound
Waters by Molecular Mechanics
Yoshiro NAKATA*, Akira TAKAHASHI*1 and Toshiharu TAKIZAWA*1
 Return
Return
Introduction
In phospholipids-water systems, head groups of lipid molecules have been
known to reorientate in the surface of the bilayer plane with the correlation
time of the order of 1 ns[1].
It is also known that several water molecules
are bound to the phosphate group in the head group with a lifetime much
greater than the reorientation correlation time of the head
group[2]. These
facts have suggested that hydrated water molecules may be kept tightly
bound to the head group while it is rotating. Recently we have obtained
direct evidence for this by the study of the spin-lattice relaxation for the
proton of water(HDO) bound to the head group in dipalmitoylphosphatidyl-
choline(DPPC)-D2O system[3].
In this paper, we report the favorable sites for hydration of the phosphate
group in the phosphatidylcholine head group calculated by molecular
mechanics.
Methods
The MMHS-ex (Molecular Model Handling System expanded edition)
software system[4,5] was used
to carry out the model building and the
molecular mechanics calculations.
Configuration of phosphatidylcholine head group;The bond distances
and bond angles were taken from the crystal X-ray structures of glyceryl-
phophorylethanolamine(GPE) and
glycerylphosphorylcholine(GPC)[6,7]. The
torsional angles used are a1=180, a2=60, a3=65, a4=180, and a5=300
according to the conformation of the crystallographic date of GPC. The trans
conformation about a4 occurs for strong hydration of the phosphate
group[8,9].
The head group is assumed to be rotating around the glycerol
C2-C3 axis, which is almost perpendicular to the bilayer plane.
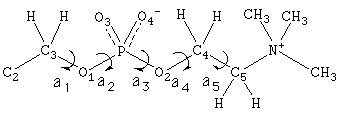
Figure 1. Chemical Structure of phosphatidylcholine head group
and torsional rotation angle definitions.
Hexagonal arrangement of phosphatidylcholine head groups in a
bilayer plane; It is assumed that the lipid molecules are packed in a
hexagonal lattice, and that each lipid head group reorients randomly about
the bilayer normal. The distance between neighboring molecules is 8.7A,
which gives the surface area of 76A2 per molecule at a temperature near the
main transition in the La phase for DPPC[10].
Two head molecules were placed in distance 8.7A as the same orientation
in same plane. Then the intermolecular energy scan was performed by
simultaneously varying C2-C3 bonds from 0 to 360 at 5 intervals. The
minimum energy orientation was selected in Fig. 2 as the state I.
The state
II and III were obtained by rotating two molecules to 60 and 120
simultaneously around the C2-C3 bonds.
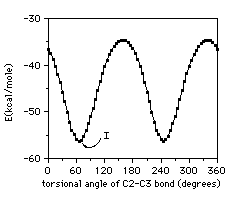
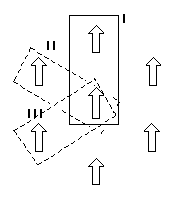
Figure 2. The calculated potential Figure 3. Hexagonal arrangement
function around C2-C3 bonds model of phosphatidylcholine
in two head group system. head groups.
Results and Discussion
Conformation Study of the favorable sites for hydration of the
phosphate group in the head group at the hexagonal arrangement;
One water molecule is placed in the position where the distance between H-
atom in water and O-atom in phosphate group is 1.45A and the angle
between P=O bond and O---H directions is 110. Then the conformation scan
was performed by varying P=O bond from 0 to 360 at 5 intervals. Five
favorable sites, resulting in minimum or lower energy, were selected, and
separated from each other in rotational angle by 120. The calculated
rotational functions are shown in Figure 4a and
4b.
The result shows that there are three favorable hexagonal arrangements of
the head groups, in which five water molecules can be placed around each
phosphate group. The most favorable arrangement of head groups viewed
perpendicular to the layer surface is given in Figure 5.
The water molecules
are drawn only about the central head group. The others are obtained by
rotating all the head groups simultaneously about each rotation axis towards
the same direction by the angle of 120 or -120.
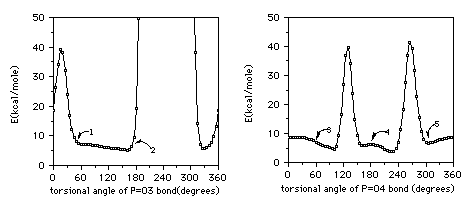
Figure 4a. The calculated potential Figure 4b. The calculated potential
function around P=O3 bond. function around P=O4 bond.
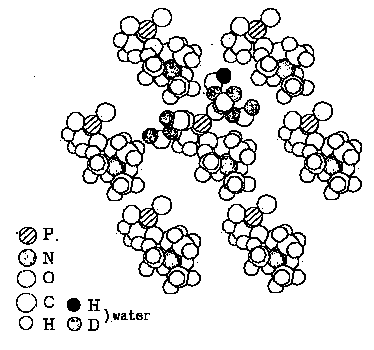
Figure 5. A hexagonal arrangement of the phosphatidylcholine
head groups model.
We have studied experimentally the spin-lattice relaxation for the proton
of water(HDO) bound to the head group in dipalmitoylphosphatidylcholine
(DPPC)-D2O system. Based on the preferred conformations and orientations
of the head groups, the relaxation rate of bound water can be calculated,
which seems fairly agreeable to the experimental value[11].
References
1) J. Seelig, L. Tamm, L. Hymel and S. Fleischer, Biochemistry, 20, p3922,
1981.
2) A. M. Gottlieb, P. T. Inglefield and Y. Lannge, Biochim. Biophys. Acta., 307,
p444, 1973.
3) A. Takahashi, T. Takizawa and Y. Nakata, Chem. Phys. Letters, 163, p65,
1989.
4) Y. Nakata, Gunma Journal of Liberal Arts and Sciences, 21, p43, 1987.
5) Y. Nakata and K. Fujinuma, Seitai Bunshi no Rittai Sekkei(in Japanese),
Saiensu Hausu, 1991.
6) G. T. Titta and B. M. Craven, Acta Crystallogr., B21, p1354, 1973.
7) R. Abrahamsson and I. Paschen, Acta Crystallogr., 21, p79, 1966.
8) M. Sundaralingam, Ann. N. Y. Acad. Sci., 195, p324, 1972.
9) B. Pullman, H. Berthod and N. Gresh, FEBS Letters, 53, p199, 1975
10) M. J. Janiak, D. M. Small and G. G. Shipley, J. Biol. Chem., 254, p6068,
1979.
11) A. Takahashi, T. Takazawa and Y. Nakata, 10th International Biophysics
Congress, Vancouver, July 29-August 3, 1990.
 Return
Return

 Return
Return

 Return
Return