Visualization of Electrochemical Measurements under Finite Conditions using JAVA and Its Application for Assisted Learning (2)
Hidenobu SHIROISHI, Toshifumi SHOJI, Tomoyo NOMURA, Sumio TOKITA and Masao KANEKO
 Return
Return
1 Introduction
The processing capabilities of a personal computer increased by two orders of magnitude in the last decade. Today a personal computer is capable of doing many jobs which could be processed only by a workstation before. Theoretical calculation using a personal computer is widely applied to the analysis of experimental data, e.g., chemical equilibrium, the rate of reaction and molecular orbital calculation.
Functional molecules incorporated into a polymer film coated on an electrode have been investigated during the last twenty years [1 - 4] for wide applications such as chemical sensors [5], electrocatalyses [6] and energy conversion devices [7]. Studies of charge propagation in a polymer membrane are indispensable for developing high-performance devices.
We have made the program, called ES-1, which can simulate an electrochemical behavior using an electrode with a finite diffusion layer under diffusion rate-limiting step considering a catalytic reaction [8]. When the interaction between the electrode and the functional molecule is weak, it is possible that a charge injection from the electrode to the molecule is the rate-determining step. ES-1 cannot simulate electrochemical behavior under such a condition. It is also difficult to solve the differential equation analytically because the ratio of the reduced molecule concentration to the oxidized one differs from that calculated from the Nernst equation.
From an educational point of view, a dynamic textbook can be made by using the program written in JAVA language because JAVA has the advantage of the affinity for HTML. Construction of such a textbook on the internet has a great significance for the application of information technologies (IT) to university education and lifelong education.
In the present paper, electrochemical behavior considering a finite diffusion layer and the reaction rate between the electrode and the functional molecule confined in a polymer membrane was simulated using JAVA language. A dynamic textbook of electrochemistry was constructed with ES-2 and HTML text.
2 Method
2. 1 Theory of an Electrochemical Simulation
The concept of the simulation is shown in Figure 1. An electrode is coated with a finite polymer layer in which functional molecules are dispersed randomly. The electrode is dipped in an electrolyte solution. Electrochemical measurement is performed by a conventional three-electrode system.
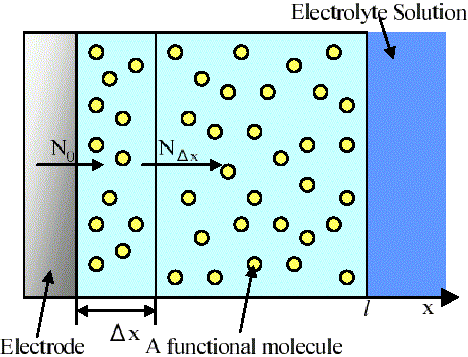
Figure 1. A concept of the electrochemical simulation. The area of the electrode is S cm2.
(i) Molar flux in the immediate neighborhood of the electrode
First, charge is injected from the electrode to a reduced molecule in the micro-volume (SDx). The injected charge raises the fraction of the oxidized molecules (RCT). The concentration of an oxidized molecule in the micro-volume is reduced by diffusion of the molecules or by a catalytic reaction. Total material balance in the micro-volume (SDx) in contact with the electrode is represented as
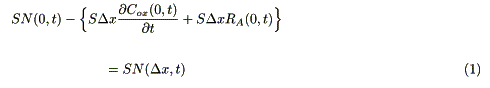
where S (cm2) is the area of the electrode, N(x, t) (mol cm-2s-1) is the molar flux of charge (or the oxidized molecules), Cox(x, t) (mol cm-3) is the concentration of the oxidized molecule, RA(x, t) (mol cm-3s-1) is the rate of the catalytic reaction.
Assuming that convection flux is negligible, N(Dx, t) is expressed by Fick's law as

A redox reaction between the electrode and the functional molecule is shown by eq. 3

where k1 (cm s-1) and k2 (cm s-1) are the rate constants for the reduction and the oxidation of the functional molecules by the electrode, respectively. These constants can be expressed as functions of electrode potential [9]:
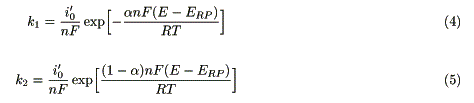
where i0' (Amol-1cm) is the exchange current density at 1 mol cm-3, n is the charge number of the electrode reaction, a is the transfer coefficient, ERP (V) is the redox potential of the molecule, and E (V) is the applied potential.
A molar flux from the electrode is represented as
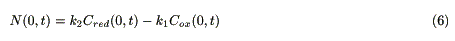
where Cred(0, t) (mol cm-3) is the concentration of the reduced molecules. A finite difference equation is derived from eqs. 1, 2 and 6, and used for the simulation [10].
(ii)Diffusion of charge in the bulk of the polymer layer
A diffusion equation in the bulk of the polymer layer is expressed as
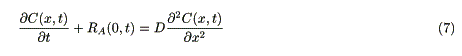
A boundary condition at the interface between the polymer layer and the electrolyte solution is represented as

where l is the thickness of the polymer layer.
2. 2 Implementation
We used a PC-9821 machine (NEC) with Microsoft Windows 2000. Microsoft Visual J++ version 6(SP3) was installed for developing ES-2. However, Windows Foundation Class library (WFC) was not used to keep a platform-independent feature. ES-2 was tested using PC-9821, IBM/AT compatible, and Macintosh with Internet Explorer and Netscape Navigator.
3 Results and Discussion
3. 1 The Feature of ES-2
The combination of ES-2 and electrochemical text written in HTML is shown in Figure 2 [11]. The parameters used for a simulation are shown in Table 1. The feature of ES-2 is similar to that of ES-1. ES-2 has only one button in the control panel for simple operation. A text of electrochemistry written in HTML containing ES-2 can be used for a dynamic textbook. ES-2 shows a voltammogram (I-V curve) and concentration distribution at a series of voltages in the layer in CV mode or a current-time curve and time dependence of concentration distribution in the layer in the potential-step mode (PS mode), and a text of current values, the fraction of the oxidized molecules and parameters used for the simulation. The results in the text area can be copied by using a shortcut key ([Ctrl]+[C]). Table 2 shows the simulation times in various environments.
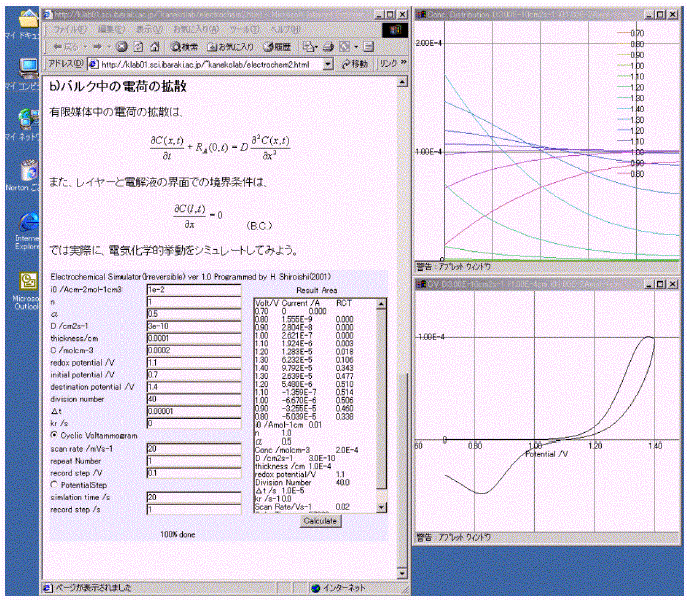
Figure 2. Combination of the ES-2 and electrochemical texts written in HTML.
Table 1. Parameters used for the siulation.
| Caption in ES-2 | | restriction |
|---|
| i0'/Acm-2mol-1cm3 | exchange current density | i0' > 0 |
| n | the number of electrions | n > 0 |
| a | transfer coefficient | 0 < a < 1 |
| D / cm2s-1 | diffusion coefficient | D > 0 |
| thickness / cm | layer thickness | thickness > 0 |
| C / mol cm-3 | concentration of a material | C > 0 |
| redox potential / V | redox potential of a material | |
| initial potential / V | potential induced at initial time | |
| destination potential / V | maximum potential | |
| division number | the division number used in the finite difference method | 0 < Div. Num. < 200, integer |
| Dt | time step used in the finite difference method | Dt > 0 |
| kr / s | first-order rate constant of catalysis | kr >= 0 |
| CV mode | | |
| scan rate / mV s-1 | scan rate per second | scan rate > 0 |
| repeat number | cycle number | repear number > 0, integer |
| record step / V | the voltage by which the results are recorded as text and concentration distribution | record step > 0 |
| Potential Step mode | | |
| simulation time / s | | simulation time > 0 |
| record step / s | the time by which the results are reccorded as text and concentration distribution | record step > 0 |
Table 2. Simulation times in various environments using default parametersa
| CPU | machine | OS | browser | Time / s |
|---|
| CV modeb | Pentium 90MHz | PC9821Xa | Windows 95 | Internet Explorer 4.0 | 106 |
| Pentium III 600MHz | IBM PC/AT Compatible | Windows 2000 | Netscape Navigator 4.7 | 14 |
| Athlon 800 MHz | IBM PC/AT Compatible | Windows 2000 | Internet Explorer 5.5 | 9 |
| Power PC G4 400MHz | Macintosh | Mac OS 8.6 | Internet Explerer 4.5 | 17 |
| PS modec | Pentium 90 MHz | PC9821Xa | Windows 95 | Internet Explorer | 359 |
| Pentium III 600 MHz | IBM PC/AT Compatible | Windows 2000 | Netscape Navigator 4.7 | 48 |
| Athlon 800 MHz | IBM PC/AT Compatible | Windows 2000 | Internet Explorer 5.5 | 32 |
| Power PC G4 400MHz | Macintosh | Mac OS 8.6 | Internet Explorer 4.5 | 60 |
a i0'=0.01A mol-1cm, n=1, C=2×10-4mol cm-3, D=3×10-10cm2s-1, thickness=1×10-4cm, division number=40, Dt=1×10-5s, kr=0 s-1b repeat number=1, scan rate=20mV/sc simulation time=20s
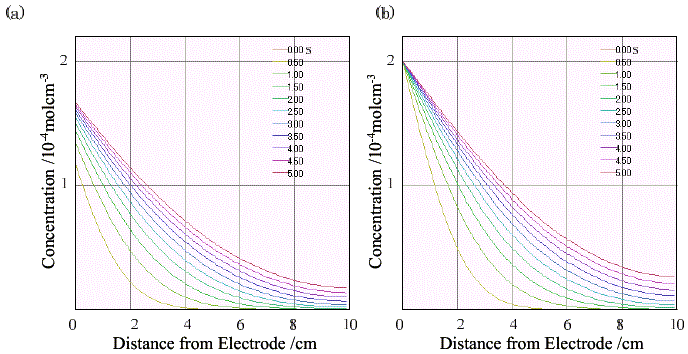
Figure 3. Time dependence of the concentration distribution of a molecule (a= 0.5, D = 3.0×10-10 cm2s-1, l = 1.0 × 10-4cm, k = 0 s-1, ERP = 1.1V vs. standard electrode) at a potential-step measurement from 0.7V to 1.5V. (a) i0' = 1.0 × 10-3 Amol-1cm (b) i0' = 1 Amol-1cm.
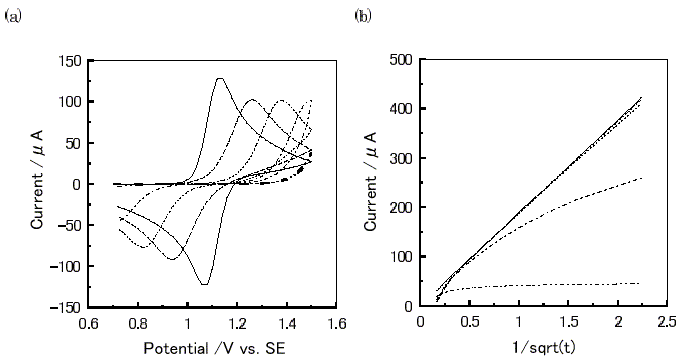
Figure 4. Virtual electrochemical measurements of a molecule (a= 0.5, D = 3.0×10-10cm2s-1, k = 0 s-1, ERP = 1.1 V vs. standard electrode) at various i0' values using ES-2. -, Nernstian Condition; ---, i0' = 1.0 ×10-1 Amol-1cm ; ..., i0' = 1.0 ×10-2 Amol-1cm ; -.-, i0' = 1.0 ×10-3 Amol-1cm ; -..-, i0' = 1.0 ×10-4Amol-1cm (a) Cyclic voltammogram between 0.7V to 1.5V at 20mVs-1. (b) Cottrell plots at the potential-step from 0.7V to 1.5V.
3. 2 The Result of the Simulation
The time dependence of the concentration distribution at a potential-step measurement is shown in Figure 3. The concentration of the oxidized molecule near the electrode increased gradually at i0' = 1.0 ×10-3 Amol-1cm (Figure 3(a)) after the potential at which a reduced molecule is oxidized completely under the Nernstian condition was applied, while the curve of the oxidized molecule rose immediately at 1 Amol-1cm (Figure 3(b)).
Figure 4(a) shows a series of cyclic voltammograms at various i0' values. The decrease of the i0' value increased the potential difference between the anodic and cathodic peaks, and at the same time their peak current decreased.
Cottrell plots at various i0' values are shown in Figure 4(b). The current at the initial time reduced with decreasing i0' value. Further decrease of the i0' value causes a constant current value in the initial time region.
4 Conclusion
A virtual electrochemical simulator, called ES-2, written in JAVA language, was developed. ES-2 can simulate potential-step measurement and cyclicvoltammetry under finite conditions by considering the rate of charge injection from the electrode to the functional molecule and the diffusion of charges. The current value at the initial time reduced with decreasing i0' value.
The authors acknowledge a Grant-in-Aid for the JAERI's Nuclear Research Promotion Program (JANP) from the Japan Atomic Energy Research Institute.
References
[ 1] C. R. Martin, I. Rubinstein, and A. J. Bard, J. Am. Chem. Soc., 104, 4817 (1982).
[ 2] W. J. Vining and T. J. Meyer, Inorg. Chem., 25, 2023 (1986).
[ 3] F. C. Anson, D. N. Blauch, J. M. Saveant, J. Am. Chem. Soc., 113, 1922 (1991).
[ 4] J. W. Long, C. S. Velazquez, and R. W. Murray, J. Phys. Chem., 100, 5492 (1996).
[ 5] G. E. Benedetto, F. Palmisano and P. G. Zambonin, Biosensors & Bioelectronics, 11, 1001 (1996).
[ 6] M. Yagi, K. Kinoshita, M. Kaneko, J. Phys. Chem., 100, 11098 (1996).
[ 7] M. Kaneko, Photoelectric Conversion by Polymeric and Organic Materials, H. S. Nalwa ed., Organic Conductive Molecules and Polymers, 4, John Wiley & Sons, Ltd. (1997), 669.
[ 8] The following article corresponds to "Visualization of Electrochemcial Measurement under Finite Conditions using JAVA and it's application for Assisted Learning (1)"
H. Shiroishi, T. Nomura, K. Ishikawa, S. Tokita and M. Kaneko, J. Chem. Software, 7, 145 (2001).
[ 9] A. Fujishima, M. Aizawa, T. Inoue, Denkikagakusouteihou, Gihoudou Syuppan Co., Ltd. (1984).
[10] ES-2 simulates the electrochemical measurement by the forward difference method. In large concentration gradient near the electrode, a larger division number is desirable for an accurate simulation. For large diffusion coefficient or large k value, it is preferable that a small Dt be used for a correct simulation. ES-2 and ES-1 with Crank-Nicolson method are also available at the same addresses.
[11] This program incorporated into an electrochemical text written in HTML can be seen at http://klab01.sci.ibaraki.ac.jp/~kanekolab/electrochem2.html
 Return
Return

 Return
Return
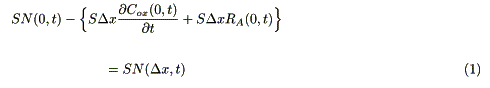


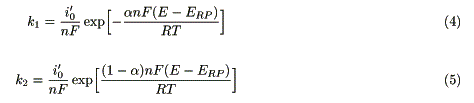
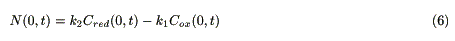
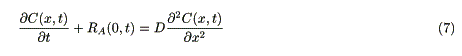




 Return
Return